Helium,
the smallest atom on the periodic table, has many interesting
properties. For one, it will not solidify unless pressurized
to an excess of twenty five atmospheres. At normal pressures,
helium will remain a liquid all the way down to zero degrees
Kelvin. This is the theoretical lowest possible temperature,
and is known as Absolute Zero. Although it will not solidify,
liquid helium does go through a phase change at 2.17 degrees
Kelvin (-456°F/-271°C). This is known as the lambda
point temperature. Above the lambda point liquid helium behaves
as any other liquid, but below the lambda point some of its
atoms become superfluid. These atoms are in their
lowest possible energy state, so they have no entropy and
no viscosity, which means that they do not interact at all
with other atoms. This allows the atoms to flow freely without
any drag or friction. We distinguish these two states by
calling the normal liquid helium I, and below the
lambda point calling the liquid helium II.
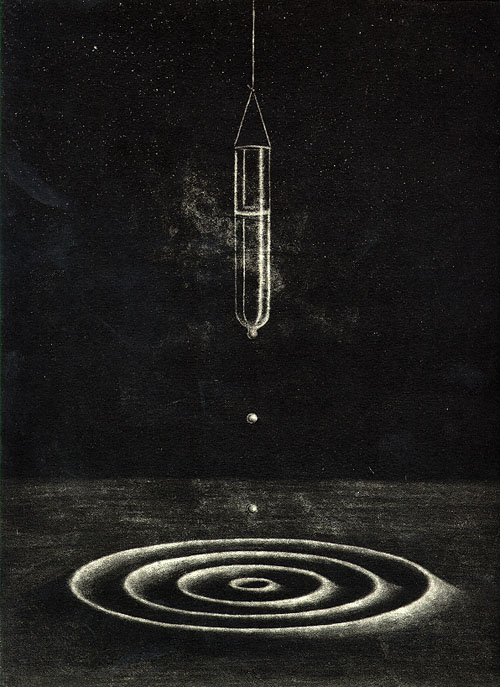
My print depicts one experiment performed
with liquid helium, in which a test tube is filled with helium
II by dipping it into a large helium bath and then dangling
it above the surface of the bath by a fine thread. When this
is done, a very thin layer of normal helium atoms (perhaps
a few atoms thick at most) will stick to the surface of the
test tube. This film creates small channels through which
the superfluid helium atoms can flow. They do not stick to
the glass because they have no viscosity. The superfluid
helium on the outside of the test tube will flow down its
surface and drip back into the helium bath (seeking the lowest
possible energy level in earth's gravity). As it does this,
the small channels act like little siphons. Just as you can
siphon the gas out of a car using a small hose, the act of
the superfluid helium flowing through these channels pulls
more helium up over the edges of the test tube, which in
turn drips back down to the helium bath, siphoning more helium,
etc. Given enough time the helium will siphon itself completely
out of the test tube leaving it empty. If the test tube is
left partially submerged in the helium bath, then the fluid
will flow out until the liquid level in the test tube is
even with the level of the helium bath. Likewise, if the
liquid level in the test tube is lower then that of the bath,
then the helium will flow into the test tube to equalize
the levels.
|
 |
 |

![]()
